|


Register for the
Painsley Small
Worlds Award.
Why not join our
mailing list?
Contact us.

| |
Two temperature sensors can collect some interesting information about a change of
state during cooling. In this experiment the temperature of a water bath increases as a
substance cools. The setup will also show that, during the change of state, heat is lost
from the substance even though its temperature remains constant.
Setting up
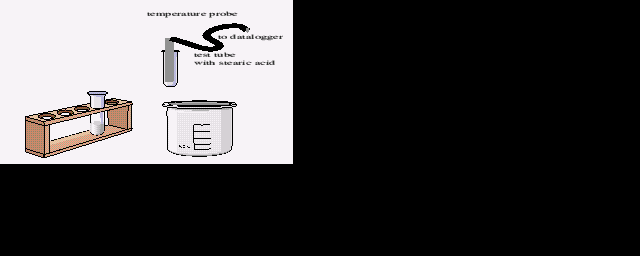
 | Connect two temperature sensors to channels 1 and 2 on the interface. |
 | Place one temperature probe in a test tube half-filled with stearic acid. |
 | Warm the tube and probe in a water bath to melt the stearic acid.
|
Apparatus
 | Beaker |
 | test tube |
 | water bath |
 | insulation material for the beaker |
 | stearic acid (or wax; benzophenone) |
 | test tube rack |
 | interface |
 | temperature sensors
|
Sensor identification
The software needs to know that you have connected two temperature sensors. Set them on
their 0-100 range if they are adjustable. Some systems identify sensors automatically.
Recording the data
 | Remove the tube from the water bath. |
 | Place it in a small insulated beaker, partly filled with water. |
 | Record for 20 minutes. |
 | Stir the stearic acid continuously. |
Using the results
 | How does the graph show you that the stearic acid is getting cooler? |
 | What happening to the water as this occurs? |
 | Why is this? |
 | What is happening to the stearic acid when the graph is flatter? |
 | What happening to the water as this occurs? |
 | Where does the water gain its heat from? |
The IT in Science book of Datalogging and Control, R. Frost

Back to top
|